The safety of medical power supplies is not optional—it’s a mandatory foundation for trust in healthcare devices.
Medical power supplies must meet IEC60601 standards and be certified by recognized bodies like UL or TUV to ensure patient and equipment safety.
To protect both patients and medical staff, power supplies used in healthcare settings must follow strict safety standards. These standards are not just checkboxes—they are detailed systems designed to eliminate risk and ensure reliability. If you’re designing or selecting power supplies for medical use, understanding these standards helps prevent regulatory pitfalls and build safer, more competitive products.
What is the IEC60601 series, and why is it important?
No matter how advanced your equipment is, without meeting IEC60601 standards, it’s not ready for the medical market.
The IEC60601 series outlines mandatory safety and performance requirements for all medical electrical equipment, including power supplies.
Dive Deeper: Breaking Down the IEC60601 Series
The IEC60601 standard series forms the backbone of medical device safety across the world. It’s not a single document but a framework of several key parts, each covering a specific area of concern. Here’s a breakdown:
| IEC60601 Part | Focus Area | Key Requirement |
|---|---|---|
| IEC60601-1 | General safety | Electrical, mechanical, and thermal safety |
| IEC60601-1-2 | Electromagnetic compatibility (EMC) | Immunity to and emissions of electromagnetic noise |
| IEC60601-1-4 | Software life cycle | Safe software design and maintenance practices |
| IEC60601-1-6 | Usability | Human factor design for reduced operational risk |
| IEC60601-1-8 | Alarm systems | Clear, standardized alarms for urgent situations |
For medical power supplies, compliance with IEC60601-1 is the most critical. It covers everything from leakage current limits to proper insulation and grounding methods. It’s not just about protecting the patient, but also about preventing device failure that could lead to misdiagnosis or injury.
In my own work with OEMs, failing early EMC pre-compliance tests under 60601-1-2 was one of the most common roadblocks. Designing with these requirements in mind saves massive time and cost later.
Who provides certification for medical power supplies?
Meeting standards is only half the battle. You also need someone to verify you’ve done it right.
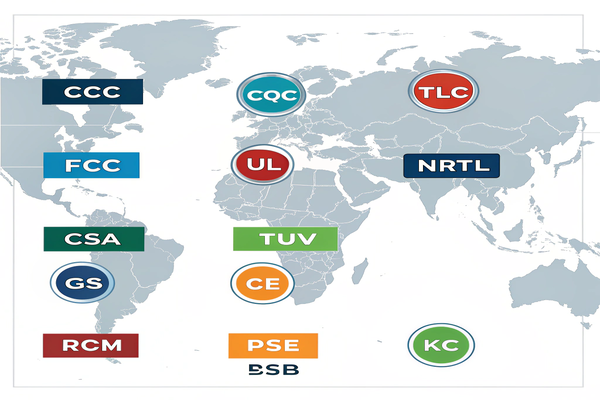
Trusted certification bodies like UL, TUV, or CSA test and confirm that a medical power supply complies with safety standards.
Dive Deeper: Why Third-Party Certification Matters
Getting certified by an independent organization gives you credibility. Here are the most common names in medical device testing:
| Certifier | Country Origin | Typical Focus Area |
|---|---|---|
| UL (Underwriters Laboratories) | United States | Electrical and fire safety |
| TUV (Technischer Überwachungsverein) | Germany | Mechanical, EMC, and environmental compliance |
| CSA (Canadian Standards Association) | Canada | Electrical and performance testing |
| Intertek | Global | Wide-range compliance, including RoHS and energy |
Each organization follows similar testing protocols based on IEC standards. For many global manufacturers I work with, the choice of certifier depends on the destination market. North America tends to prefer UL or Intertek, while Europe leans toward TUV.
But certification is not a one-time event. These bodies also conduct periodic audits. You need to be ready at any time to prove your process is still under control.
What safety design measures should be built into power supplies?
Standards and certification matter, but real safety starts with smart design.
Design measures like insulation, short-circuit protection, and secure grounding form the foundation of a safe medical power supply.
Dive Deeper: Key Safety Features You Can’t Ignore
In the early design phase, incorporating proper safety measures prevents costly redesigns. Here are some essential ones:
| Safety Feature | Function |
|---|---|
| Double insulation | Prevents shock in case of a single insulation fault |
| Overload/Short-circuit protection | Protects both the power supply and connected device |
| Leakage current control | Ensures safe current levels through the patient |
| Grounding | Reduces risk of accidental electrical discharge |
| Fire-resistant casing | Prevents spread of fire in case of internal failure |
In one project, a client underestimated the space needed for proper creepage and clearance. As a result, the initial prototype failed the high-voltage test and had to be redesigned. This is why careful planning in early electrical layout and PCB design is critical when targeting IEC60601.
How do you maintain safety after certification?
Getting certified is the start. Keeping that level of safety is the real challenge.
Continuous monitoring, testing, and feedback collection are essential to maintaining long-term product safety.
Dive Deeper: Safety Doesn’t Stop at Approval
Once your adapter is certified and shipped, the job isn’t done. Here’s what ongoing safety management looks like:
| Process | Purpose |
|---|---|
| Incoming material inspection | Ensures component consistency from suppliers |
| In-line and final testing | Detects assembly issues before shipment |
| Field feedback tracking | Flags issues experienced by users in real environments |
| Failure analysis (FA) | Investigates root causes of defects |
| Regular audits | Maintains certified quality levels over time |
Some of our longest-standing customers chose us not just because we passed the initial test, but because we consistently delivered products that met spec every time. That kind of reliability only comes from strict quality loops and commitment to safety at every level—from R&D to shipping.
Conclusion
Safety standards like IEC60601 and certification systems ensure medical power supplies protect both patients and professionals. They are essential from design to long-term use.